Imagine stepping onto an empty dance floor. You’re free to move, spin, and spread out. Now imagine that same dance floor during a sold-out concert – packed shoulder-to-shoulder, every step a collision. This striking contrast perfectly mirrors the invisible world of gases, a world that puzzled and fascinated scientists for centuries. How does something that seems like “nothing” exert immense pressure or power mighty engines? This profound question ignited a scientific investigation, transforming our understanding of the universe one invisible particle at a time.
The Great Search: Finding what we cannot see
For centuries, the nature of air was a profound mystery. Ancient Greek philosophers believed air was a fundamental element, a continuous substance, and the idea of a vacuum was considered impossible. These deep-rooted misconceptions hindered progress for nearly two thousand years.
The true journey began with everyday observations. Why could a suction pump only lift water to a certain height? In the early 17th century, Galileo Galilei questioned this limit, suspecting that the “force” pulling water up was actually the weight of the air pushing down. He couldn’t quite prove it, but his skepticism opened the door.
The real breakthrough came in Italy, around 1643, with Galileo’s student, Evangelista Torricelli. He designed a brilliant experiment: filling a long glass tube with mercury, inverting it into a dish, and observing the mercury column settling at about 76 cm, leaving an empty space (a vacuum!) above it. His key discovery that the mercury was held up not by suction, but by the weight of the air pressing down on the mercury in the dish. He had not only created the first sustained vacuum but also invented the barometer and quantified atmospheric pressure.
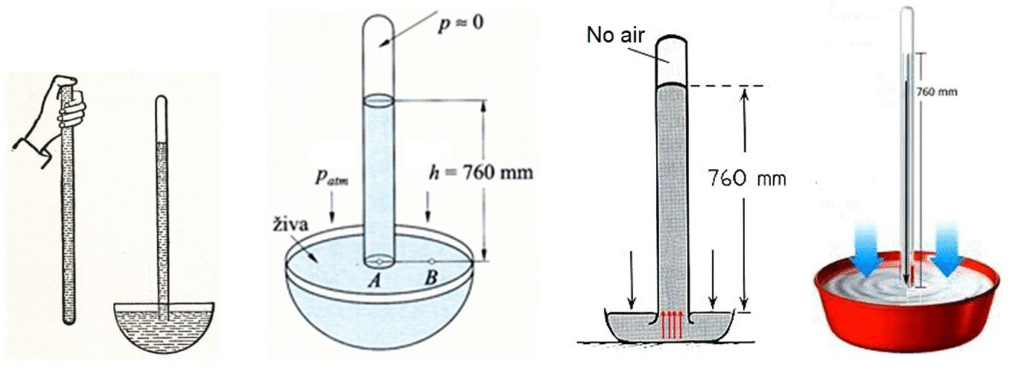
A decade later, in France, Blaise Pascal took Torricelli’s work to new heights, literally. In 1648, he arranged for his brother-in-law to carry a barometer up the Puy de Dôme mountain. As expected, the mercury column dropped at higher altitudes, definitively proving that air had weight and that its pressure varied. The “horror vacui” was disproved, replaced by the simple concept of a sea of air exerting pressure.
The stage was set for understanding how gases behave. In the 1660s, in Oxford, England, Robert Boyle, a brilliant natural philosopher, built an improved air pump and conducted rigorous experiments. He carefully measured the relationship between the volume of air and the pressure it exerted, showing that as you compress a gas, its pressure increases proportionally. This fundamental relationship is now known as Boyle’s Law ( P ∝ 1/V). Boyle was also among the first to propose that air consisted of tiny, springy particles—a truly revolutionary idea for his time.
Fast forward to the late 18th century. In France, Jacques Charles (1787) and Joseph Gay-Lussac (1802) explored the relationship between gas volume, temperature, and pressure. Charles discovered that gases expand when heated, a principle quickly put to use in hot air balloons and now known as Charles’s Law ( V ∝ T ). Gay-Lussac also observed a simple whole-number ratio in the volumes of reacting gases, hinting at an underlying particulate structure.
The idea really grew with Amedeo Avogadro in Italy (1811), who proposed that equal volumes of all gases, at the same temperature and pressure, contain the same number of molecules (Avogadro’s Law). This was a monumental leap, clarifying the difference between atoms and molecules and providing a way to “count” these invisible entities by relating the number of moles to the number of particles.
The Breakthrough: A World of Dancing Particles
But what were these particles doing? The true “dance floor” came alive with the Kinetic Theory of Gases. Daniel Bernoulli, in the early 18th century, had an early intuition, describing gases as particles in constant, chaotic motion, colliding with each other and the container walls. However, his ideas lay dormant until the mid-19th century when giants like Rudolf Clausius, James Clerk Maxwell, and Ludwig Boltzmann resurrected and rigorously developed this theory.
Their brilliant model, now the foundation for understanding what we call an Ideal Gas, proposed that:
- Pressure isn’t some abstract force; it’s the sum of countless tiny impacts as these particles bounce off the container walls. More collisions, or more forceful collisions, mean higher pressure.
- Temperature isn’t just “hotness”; it’s a direct measure of the average kinetic energy (speed) of these particles. Hotter gas means faster-moving particles.
This elegantly simple model, describing our ’empty dance floor’ scenario, brilliantly connected the visible world of gas behavior to the invisible world of atomic motion.
🤔 Challenge Your Intuition…
Imagine you have an ideal gas in a sealed container with a movable piston, like a syringe. You slowly push the piston in, reducing the volume of the gas to half, while keeping the temperature constant.
According to Boyle’s Law, the pressure should double. But does this mean the average kinetic energy of the gas molecules has also doubled, making them move twice as fast?
The Misconception: It’s tempting to think that increased pressure must mean faster-moving particles! However, recall Boyle’s Law states P∝1/V at constant temperature. If the temperature is constant, then according to the Kinetic Theory, the average kinetic energy of the molecules must also remain constant. Thus, the pressure increases not because the particles are moving faster, but because they are hitting the container walls more frequently due to being crammed into a smaller volume.
This subtle distinction, which Boyle and later Boltzmann elucidated, is crucial for understanding the Kinetic Model’s assumptions and avoiding misconceptions about how pressure and temperature are related.
To truly grasp how these concepts interrelate in real problems, watch this video: (This video will help students understand how pressure changes without a change in kinetic energy when temperature is constant.)
The Reality Check: When the Dance Floor Becomes an Elevator
While the Ideal Gas model, a triumph of classical physics developed by Clausius, Maxwell, and Boltzmann, provides incredibly accurate predictions for most scenarios, these very scientists understood its limits. The ideal model assumes:
- Gas particles themselves have no volume (they are point masses).
- There are no attractive or repulsive forces between particles.
This is our perfect ’empty dance floor.’ But what happens when we force the dancers into a ‘crowded elevator’—that is, when we look at Real Gases? This challenge was later systematically addressed by scientists like Johannes van der Waals.
At very high pressures (cramming them together) or very low temperatures (slowing them down), those ideal assumptions break down:
- Molecular Volume Matters: The actual volume of the particles is no longer negligible compared to the total volume of the container. The particles themselves take up noticeable space.
- Intermolecular Forces Emerge: When particles get very close and slow down, faint attractive forces between them (like dancers bumping into each other) start to have a significant effect, pulling them closer and reducing the pressure they exert on the walls.
🤔 Another Tricky Question for You…
You’re studying a sample of real oxygen gas. You want this real gas to behave as much like an ideal gas as possible, aligning with the model conceptualized by Clausius and Maxwell.
What conditions should you impose? Should you place it in a very small container and cool it down significantly, or put it in a very large container and heat it up a lot?
The Misconception: This question challenges a very common misconception. A “small container and cool it down” scenario sounds like it might “tame” the gas. However, the exact opposite is true! Cooling the gas down and compressing it (small container means high pressure) forces molecules closer together and slows them down. This enhances the effects of molecular volume and intermolecular forces, making the real gas deviate more from ideal behavior.
Understanding the conditions where real gases approximate ideal gases is key to applying the gas laws correctly, just as van der Waals demonstrated with his modified equation.
To unlock the conditions for ideal gas behavior and solve related problems, watch this video: (This video directly addresses the conditions for ideal behavior in real gases.)
Why It Matters: From Weather Forecasts to Space Travel
The understanding of gas behavior is not merely an academic curiosity; it’s a cornerstone of modern science and technology. The meticulous observations of Boyle in the 17th century are foundational to the design of modern engines. The theoretical work of Clausius and Boltzmann laid the groundwork for thermodynamics, essential for power generation and refrigeration. Charles’s Law is applied in meteorological models to predict weather.
From the simple act of inflating a tire to the complex calculations for launching a satellite, our mastery of gas behavior, built by generations of scientists, is a testament to scientific inquiry.
Beyond the Equations: A TOK and Nature of Science Perspective
This incredible journey of understanding gases isn’t just about memorizing laws or solving problems. It’s a fantastic case study for the Nature of Science (NOS) and raises key questions for the Theory of Knowledge (TOK):
- Models and Reality: The “Ideal Gas” model, a powerful creation of Maxwell and Boltzmann, is a simplification. It works brilliantly under certain conditions but fails in others. This highlights a core NOS theme: scientists use models to understand the world, but these models are not perfect copies of reality. It makes us ask, to what extent do scientific models give us knowledge of the natural world?
- The Human Element in Science: This story wasn’t the work of one lone genius. It was a collaborative effort spanning centuries and countries, with scientists like Galileo, Torricelli, Boyle, and Boltzmann building on, and sometimes correcting, the work of those who came before. Science is a cumulative and human endeavor, shaped by collaboration, creativity, and persistence.
- Paradigm Shifts: For nearly 2000 years, the idea of “horror vacui” was a deeply-rooted paradigm. It took the empirical evidence from Torricelli’s and Pascal’s experiments to overthrow this long-held belief. This shows how scientific knowledge can undergo radical shifts when new evidence challenges the existing framework.
- Ways of Knowing: How can we claim to know anything about particles we cannot see? This story shows the interplay between Sense Perception (observing the mercury column) and Reason (inferring the existence and behavior of invisible particles). It demonstrates how scientists use indirect evidence to build knowledge about the microscopic world.
The Journey Continues
In the end, the story of gases—from Torricelli’s humble mercury tube to Boltzmann’s grand statistical theories—is the story of science itself. It’s a a captivating narrative of human curiosity, struggle, and profound insight. It’s a powerful reminder that our understanding of the universe is built step-by-step, with each generation of curious minds asking new questions and challenging old ideas. The “Ideal Gas” model isn’t the final, perfect truth, but an incredibly useful map that helps us navigate a complex reality. This journey teaches us that science isn’t about having all the answers, but about the ongoing, exciting adventure of asking better questions. What invisible world will your curiosity help uncover next?